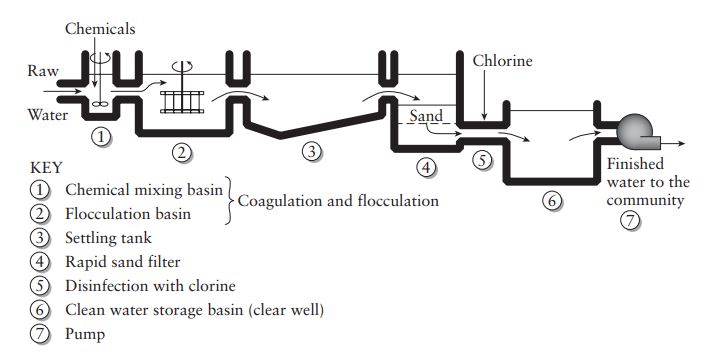
Water Treatment
Hardness is a caused due to the existence of minerals in water (calcium, magnesium, iron, ...)
Hardness is measured in united of mg/L as CaCO3 or meq/L
Calculate Concentration as CCaCO3
| Concentration C in mg/L | ||
| Atomic/Molecular Weight mg/mmol | ||
| Equivalent Weight | {{EW()}} mg/meq | |
| Concentration meq/L | {{Cq()}} meq/L | |
| Concentration as CCaCO3 | {{CCaCO3()}} mg/L |
Total Hardness
| Ca2+ | Mg2+ | Fe2+ |
| Fe3+ | Mn3+ | Sr2+ |
| Al3+ | ||
| TH {{TH()}} meq/L | TH as CaCO3 {{THCaCO3()}} mg/L |
| Initial Hardness | |
| Desired Hardness | |
| % Bypass | {{Bp()}} |
| Water Softener Resin Volume | |
| Resin Exchange Capacity | |
| Volume of Water Used | |
| Time for Regenration Cycle | {{trCycle()}} day |